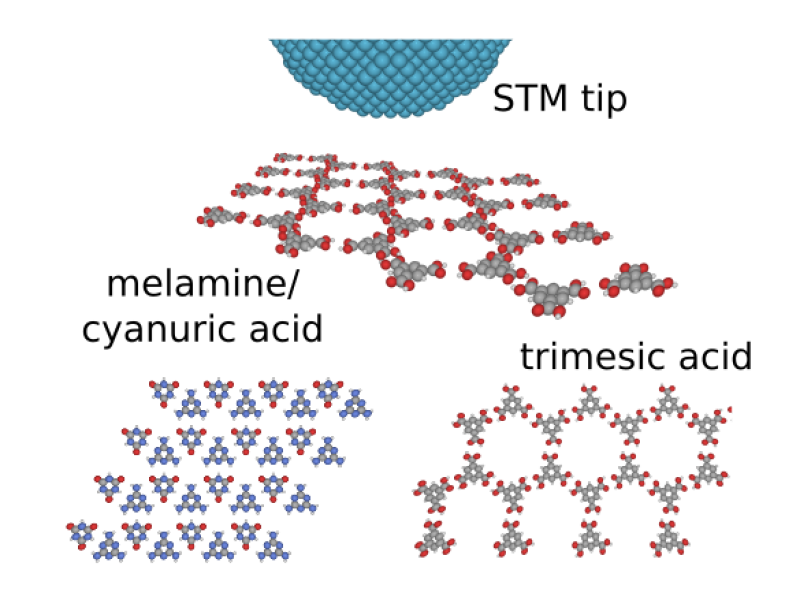
Hydrogen-bonded molecular networks studied by STM
Advisor: Pavel Kocán (MFF UK)
Funding: Fully funded
Contact: pavel.kocan@mff.cuni.cz
Future molecular nano-electronics will likely rely on the self-assembling process of organic molecules on surfaces of crystals. Hydrogen bonding between functional groups of organic molecules represents a fascinating mechanism in formation of highly ordered molecular networks [1-5]. Reversibility of the interaction and its cooperative nature allow formation of perfect supramolecular architectures. Specificity and directionality of the hydrogen bonds allow to design the assemblies by selecting suitable molecules. The same properties make this kind of interaction crucial in chemistry of living organisms – replication of DNA molecules being the most important example. Additional degree of architecture tunability can be achieved by combining two (or more) molecules, resulting in a growth of bicomponent (or higher-component) assemblies [1-5].
Impressive results in this field have been achieved mostly on metal surfaces. For electronic applications, silicon substrates would be a great benefit. The Si surfaces functionalized by metal atoms bring extra properties, such as Rashba spin-split electronic bands [6], 2D superconductivity [7] or bands with nearly massless electrons [8]. These properties can be controlled by presence of molecular layers.
Molecular self-ordering via hydrogen bonding on metal-passivated surfaces will be studied experimentally by means of the scanning tunneling microscope (STM). The samples will be prepared and examined in-situ under ultra-high vacuum conditions. As complementary techniques, theoretical simulations are expected to be used by the candidate, pair-potential-based calculations to optimize molecular structures and kinetic Monte Carlo simulations to understand mechanism of self-ordering processes.
[1] Cañas-Ventura, M. E. et al., ACS Nano 5, 457–469 (2011). [2] Xu, W. et
al., Small 3, 854–858 (2007). [3] Perdigão, L. M. A., Champness, N. R. &
Beton, P. H., Chem. Commun. 538–540 (2006) [4] Theobald, J. A., Oxtoby, N. S.,
Phillips, M. A., Champness, N. R. & Beton, P. H., Nature 424, 1029–1031
(2003).
[5] Madueno, R., Räisänen, M. T., Silien, C. & Buck, M., Nature 454,
618–621 (2008). [6] Ibañez Azpiroz, J., Eiguren, A. & Bergara, A., Phys.
Rev. B 84, 125435 (2011). [7] Zhang, T. et al., Nature Phys. 6, 104–108
(2010). [8] Kim, K. S., Jung, S. C., Kang, M. H. & Yeom, H. W., Phys. Rev.
Lett. 104, 246803 (2010).